The Future of Medical Diagnostics
By Tony Clark, 2-Dooz Inc. – May 1, 2013 (Original Publication Date)
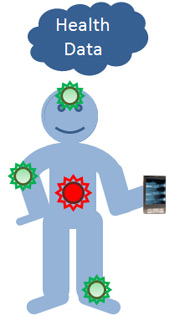
 One of my predictions from the beginning of this year calls for the accelerated development of nanotechnology based biosensors. I foresee these devices ushering in an era comprising more proactive, less invasive and potentially more cost effective medical testing and treatment. The combination of nanotech biosensors, smart phones and cloud technology will enable a number of new applications, including the remote (e.g., in-home) collection of real time medical data that can be used to facilitate diagnosis and treatment.
One of my predictions from the beginning of this year calls for the accelerated development of nanotechnology based biosensors. I foresee these devices ushering in an era comprising more proactive, less invasive and potentially more cost effective medical testing and treatment. The combination of nanotech biosensors, smart phones and cloud technology will enable a number of new applications, including the remote (e.g., in-home) collection of real time medical data that can be used to facilitate diagnosis and treatment.
The April 16, 2013 VLAB panel discussion, led by Dr. Daniel Kraft, on the future of diagnostics, provided a status update on where we currently stand regarding the realization of the above prediction and, perhaps more importantly, identified key headwinds that are inhibiting progress.
Status Check
Collectively the VLAB panel envisions a major transformation in healthcare, wherein the current system is replaced by one in which health data is collected continuously and not just when a patient presents with an illness. Multiple sensors, which are affixed to the consumer, are used to collect data. The health data is then relayed from the sensors via the consumer’s smart phone to the cloud where it is processed and stored. Continuous diagnostics enables more proactive (less reactive) treatment models, which in turn should lead to better outcomes. Regarding the status of development, sensors already exist for measuring blood sugar, heart rate, blood pressure, respiration rate, temperature, and oxygenation. Moreover, the smart phone and cloud infrastructure components of the solution are largely in place.
Consumer Driven Medicine (CDM) defined: A medical system in which consumer needs and demands—such as convenience, ease of use, cost and valuable results—drive products and services. In this system, we as consumers hold power over our own healthcare by choosing which products and services to purchase based on what we value, and by demanding the ability to record and then use our health data. This is a radical change from a “last century” health system, in which government and private insurers, healthcare providers, regulators, and technology companies drove the healthcare market and patients were mere passive participants. –-Source: www.vlab.org
Headwinds
For sure, much progress has been made. However, there are several headwinds that are impeding the widespread use of consumer biosensors. The good news is that none of the headwinds are related to the rate of innovation or to technological development challenges.
- Entrenched reimbursement models. Today’s healthcare practices and reimbursement are optimized around reactive treatment, whereby it is estimated that 20% of the population generates 80% of the healthcare costs. The Affordable Healthcare Act takes baby steps towards a more proactive treatment model, by significantly expanding preventative care at no additional cost to the subscriber. However, we have a long way to go before the on-going, proactive collection of healthcare data through sensors and continuous diagnosis is widely covered.
- Liability. Along with new testing and treatment models come new liability concerns. Placing blame is a fact of life. If the above new approaches fail to catch a preventable illness in time, who will be liable? Will liability ultimately reside with the sensor manufacturers, the creators of the diagnostic algorithms, the cloud and smart phone equipment manufacturers, the communications service providers, or with the consumer—because of poor compliance?
- FDA. The VLAB panelist referred to the FDA as the F-word. Mostly the panel members were referring to the funding gap that is created by the complexity and inherent uncertainty of the FDA approval process. A large number of ideas are being incubated, but venture capitalists are hesitant to provide the necessary follow-on funding due to the uncertainty of the regulatory process.
In summary, we are well on the way towards using biosensors to remotely collect real time medical data that can be used to facilitate diagnosis and treatment. Innovation is pervasive; and the pace appears to be accelerating. That said, regulatory, liability, and entrenched healthcare practices are headwinds that are inhibiting the widespread adoption of these promising technologies.
Those are my thoughts. And, as always, I invite and look forward to learning what you think.
